Speciation
Speciation is about how species form. It is a major part of evolutionary biology.
Darwin thought most species came directly from pre-existing species. This is called anagenesis: species by changing, or 'phyletic evolution'. For much of the 20th century, scientists thought most species came when earlier species split. This is called cladogenesis.[1][2] The general view was that most species splitting is caused or helped on its way by isolating mechanisms.[3][4]
No doubt the physical separation of species which once lived together is a main factor. It is illustrated by so many examples, some of which are discussed below.
However, work in the last 20 years has shown some other causes. Analysing the DNA sequence of living things has shown that there is often some hybridisation between related species. That means genes have been transferred by these crosses. In turn, that means reproductive isolation is not the only definition of a species, and speciation does not always need allopatry (species to be reproductively separated).[5][6][7] The sections below illustrate the idea that physical separation was of prime importance in the formation of new species.
Isolating mechanisms
[change | change source]Isolating mechanisms are things which prevent successful breeding between groups in a species. Reproductive isolation of populations is established. This is particularly important to the biological species concept, as species are usually defined by their reproductive isolation.
Isolating mechanisms can be divided into two groups, before and after fertilisation.
Before fertilisation
[change | change source]
Factors which prevent individuals from mating.
- Geographic isolation: Species occur in different areas, and are often separated by barriers.
- Temporal isolation: Individuals do not mate because they are active at different times. This may be different times of the day or different seasons. The species mating periods may not match up. Individuals do not encounter one another during either their mating periods, or at all.
- Ecological isolation: Individuals only mate in their preferred habitat. They do not encounter individuals of other species with different ecological preferences.
- Behavioral isolation: Individuals of different species may meet, but one does not recognize any sexual cues that may be given. An individual chooses a member of its own species in most cases.
- Mechanical isolation: Copulation may be attempted but transfer of sperm does not take place. The individuals may be incompatible due to size or morphology.
- Gametic incompatibility: Sperm transfer takes place, but the egg is not fertilized.
After fertilisation
[change | change source]Factors which prevent mating being successful, such as genetic incompatibility, hybrid inviability or sterility.
- Zygotic mortality: The egg is fertilized, but the zygote does not develop.
- Hybrid inviability: Hybrid embryo forms, but is not viable.
- Hybrid sterility: Hybrid is viable, but the resulting adult is sterile.
- Hybrid breakdown: First generation (F1) hybrids are viable and fertile, but further hybrid generations (F2 and backcrosses) are inviable or sterile.
Geographical isolation
[change | change source]This is thought to be the most comon cause of speciation. The first person to think of it was Moritz Wagner, a German explorer and natural historian.
Wagner's early career was as a geographer, and he published a number of geographical books about North Africa, the Middle East, and Tropical America. He was also a keen naturalist and collector, and it is for this work he is best known among biologists. Ernst Mayr, the evolutionist and historian of biology, has given an account of Wagner's significance.[8]p562–565
During his three years in Algeria, Wagner (amongst other activities) studied the flightless beetles Pimelia and Melasoma. Each genus is split into a number of species, each of which is confined to a stretch of the north coast between rivers which descend from the Atlas Mountains to the Mediterranean. As soon as one crosses a river, a different but closely related species appears.[9]
- "... an incipient species will only [arise] when a few individuals transgress the limiting borders of their range... the formation of a new race will never succeed... without a long continued separation of the colonists from the other members of their species".[10]
This was an early description of a process of one kind of geographic speciation. In 1942 it was reintroduced by Mayr,[11] and the importance of geographic speciation became one of the core ideas of the evolutionary synthesis.[12]
Another term for geographic speciation is allopatric speciation. Allopatry means 'different land'.
Vacant islands
[change | change source]Volcanic islands are formed with no life, and all life has to arrive carried by wind or water. We know from the Hawaiian islands and from the Galapagos Islands that all forms of life change when they reach the islands from the mainland.
In about 6,500 sq mi (17,000 km2), the Hawaiian Islands have the most diverse collection of drosophilid flies in the world, living from rainforests to mountain meadows. About 800 Hawaiian drosophilid species are known.
Studies show a clear "flow" of species from older to newer islands. There are also cases of colonization back to older islands, and skipping of islands, but these are much less frequent.
By potassium/argon radioactive dating, the present islands date from 0.4 million years ago (mya) (Mauna Kea) to 10mya (Necker). The oldest member of the Hawaiian archipelago still above the sea is Kure Atoll, which can be dated to 30 mya.

The archipelago itself, produced by the Pacific plate moving over a hot spot, has existed for far longer, at least into the Cretaceous. The Hawaiian islands plus former islands which are now beneath the sea make up the Hawaiian–Emperor seamount chain; and many of the underwater mountains are guyots.[13]
All of the native drosophilid species in Hawaiʻi have apparently descended from a single ancestral species that colonized the islands, about 20 million years ago. The subsequent adaptive radiation was spurred by a lack of competition and a wide variety of vacant niches. Although it would be possible for a single pregnant female to colonise an island, it is more likely to have been a group from the same species.[14][15][16][17]
There are other animals and plants on the Hawaiian archipelago which have undergone similar, if less spectacular, adaptive radiations.[18][19]
Ring species
[change | change source]
In biology, a ring species is a connected series of neighboring populations, each of which can interbreed with next-door populations. The two ends of the chain overlap.
The two end populations in the series are too distantly related to interbreed. Such non-breeding-though-genetically-connected 'end' populations may co-exist in the same region, thus closing a 'ring'.[20]
Ring species provide important evidence of evolution: they illustrate what happens over time as populations genetically diverge. Richard Dawkins observed that ring species "are only showing us in the spatial dimension something that must always happen in the time dimension".[21]

(1 : Larus argentatus argentatus, 2: Larus fuscus sensu stricto, 3 : Larus fuscus heuglini, 4 : Larus argentatus birulai, 5 : Larus argentatus vegae, 6 : Larus argentatus smithsonianus, 7 : Larus argentatus argenteus)
However, it is difficult to find a simple, straightforward example.
Larus gulls
[change | change source]This was the classic example of ring species. The range of these gulls nearly forms a ring around the North Pole (which is not normally flown over by gulls). The lesser black-backed gulls and herring gulls are sufficiently different that they do not normally hybridize; so, it was said, the group of gulls forms a continuum except where the two lineages meet in Europe.[20] However, it is generally agreed that this is not quite correct, though the details are extremely complicated.[22][23]
Ensatina salamanders
[change | change source]
Yellow: P. t. trochiloides
Orange: P. t. obscuratus
Red: P. t. plumbeitarsus
Green: P. t. "ludlowi"
Blue: P. t. viridanus
P. t. nitidus of the Caucasus is not shown.
The Ensatina salamander is a ring species in the mountains around the Californian Central Valley.[24] The complex forms a horseshoe shape around the mountains. Though interbreeding can happen between each of the 19 populations around the horseshoe, the Ensatina eschscholtzii subspecies on the western end of the horseshoe cannot interbreed with the Ensatina klauberi on the eastern end.[21] It is an illustration of "nearly all stages in a speciation process" (Dobzhansky).[24][25] Richard Highton argued that Ensatina is a case of multiple species and not a continuum of one species.[26]
The greenish warbler
[change | change source]The greenish warbler (Phylloscopus trochiloides) has a number of subspecies, of which P. t . viridianus is the most familiar in Europe. It is a ring species with populations diverging east and westwards of the Tibetan Plateau, later meeting on the northern side. Their relationships are fairly confusing.[27][28]
Sympatric speciation
[change | change source]Sympatric speciation refers to the formation of two or more descendant species from a single ancestral species all occupying the same geographic location. This is now thought to be very common.
In sympatric speciation, species diverge while inhabiting the same place. Often-cited examples of sympatric speciation are found in insects that become dependent on different host plants in the same area.[29][30]
The existence of sympatric speciation as a mechanism of speciation was hotly contested. People argued that the evidences of sympatric speciation are in fact examples of micro-geographic speciation. In general, this is not now the preferred explanation. Rather, it is the result of hybridisation between closely related species, followed by natural selection working on the offspring of such crosses. One widely accepted example of sympatric speciation is that of the cichlids of Lake Nabugabo in East Africa, which is thought to be due to sexual selection.[31]
Speciation via polyploidization
[change | change source]Polyploidy has caused many rapid speciation events because offspring of, for example, tetraploid x diploid matings often result in triploid sterile progeny.[32]
However, not all polyploids are reproductively isolated from their parental plants, and gene flow may still occur for example through triploid hybrid x diploid matings that produce tetraploids.
Many of the existing plant and most animal species have apparently undergone polyploidization in their evolutionary history.[33][34] Reproduction of successful polyploid species is sometimes asexual, by parthenogenesis. For unknown reasons many asexual organisms are polyploid.
Hawthorn fly
[change | change source]One example of evolution at work is the case of the hawthorn fly, Rhagoletis pomonella, which appears to be undergoing sympatric speciation.[35]
Different populations of hawthorn fly feed on different fruits. A distinct population emerged in North America in the 19th century some time after apples, a non-native species, were introduced. This apple-feeding population normally feeds only on apples and not on the historically preferred fruit of hawthorns. The current hawthorn feeding population does not normally feed on apples.
Some evidence suggests that sympatric speciation is occurring. Hawthorn flies mature later in the season and take longer to mature than apple flies; and there is little evidence of interbreeding (researchers have documented a 4-6% hybridization rate).
The emergence of the new hawthorn fly is an example of evolution in progress.[36]
Hybridisation
[change | change source]Rarely, a new species forms when individual members of different species mate. Usually, the products of such hybrid matings are infertile (not fertile), or relatively so, and so get eliminated by natural selection.
One example of a new and successful hybrid species has been found.[37] The new species is a hybrid of the Italian and Spanish sparrows, and the basic facts have been checked by sequence analysis from the DNA in their blood. The bird lives in Italy in a region where both parent species live. It does not reproduce with the Spanish sparrow even though it lives with them side-by-side.[37]
Artificial speciation
[change | change source]New species have been created by domesticated animal husbandry, but the initial dates and methods are not clear. For example, domestic sheep were created by hybridisation, and no longer produce viable offspring with Ovis orientalis, one species from which they are descended.[38]
Domestic cattle, on the other hand, can be considered the same species as several varieties of wild ox, gaur, yak, etc., as they readily produce fertile offspring with them.[39]
Lab species
[change | change source]The best-documented creations of new species in the laboratory were performed in the late 1980s. William Rice and G.W. Salt bred fruit flies, Drosophila melanogaster, using a maze with three different choices of habitat such as light/dark and wet/dry.
Each generation was placed into the maze, and the groups of flies that came out of two of the eight exits were set apart to breed with each other in their respective groups. After thirty-five generations, the two groups and their offspring were isolated reproductively because of their strong habitat preferences: they mated only within the areas they preferred, and so did not mate with flies that preferred the other areas.[40] The history of such attempts is described in Rice and Hostert (1993).[41]
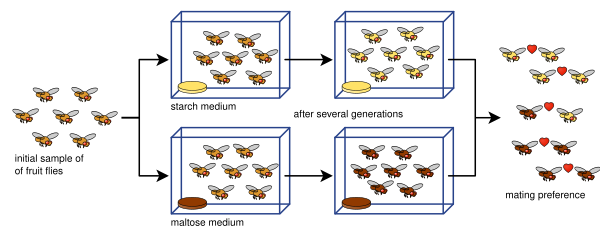
Diane Dodd was also able to show how reproductive isolation can develop from mating preferences in Drosophila pseudoobscura after only eight generations using different food types, starch and maltose.[42]
Dodd's experiment has been easy for many others to replicate, including with other kinds of fruit flies and foods.[43]
Reinforcement (Wallace effect)
[change | change source]Reinforcement is how natural selection increases reproductive isolation.[44]
It may occur after two populations of the same species are separated and then come back into contact. If their reproductive isolation was complete, then they will have already developed into two separate incompatible species.
If their reproductive isolation is incomplete, then further mating between the populations will produce hybrids, which may or may not be fertile. If the hybrids are infertile, or fertile but less fit than their ancestors, then there will be no further reproductive isolation and speciation has essentially occurred (e.g., as in horses and donkeys.)
The reasoning behind this is that if the parents of the hybrid offspring each have naturally selected traits for their own different environments, the hybrid offspring will bear traits from both, and would not fit either niche as well as either parent. The low fitness of the hybrids would cause selection to favour assortative mating,[45] which would reduce hybridization.
This is sometimes called the Wallace effect after the evolutionary biologist Alfred Russel Wallace who suggested in the late 19th century that it might be an important factor in speciation.[46]
If the hybrid offspring are more fit than their ancestors, then the populations will merge back into the same species within the area they are in contact.
Reinforcement is required for separation when there is a "hybrid zone" between two forms of a species. Hybrid zones are regions where diverged populations meet and interbreed. Hybrid offspring are very common in these regions, which are usually created by diverged species coming into secondary contact.
Without reinforcement the two populations or species would have uncontrollable interbreeding. Reinforcement may be induced in artificial selection experiments as described above.
References
[change | change source]- ↑ Cook O.F. (1906). "Factors of species-formation". Science. 23 (587): 506–507. Bibcode:1906Sci....23..506C. doi:10.1126/science.23.587.506. PMID 17789700.
- ↑ Cook O.F. (1908). "Evolution without isolation". American Naturalist. 42 (503): 727–731. doi:10.1086/279001. S2CID 84565616.
- ↑ King, David (2003). "The concept of species". Zoology 304, Evolution. Archived from the original on 2007-05-29. Retrieved 2007-07-18.
- ↑ Mallet, J.L.B. (1998). "Isolating mechanisms". Retrieved 2007-07-18.
- ↑ Mallet J. 2001. The speciation revolution. J. Evolutionary Biology 14, 887-888.
- ↑ Wang R.L; Wakeley J. & Hey J. 1997. Gene flow and natural selection in the origin of Drosophila pseudoobscura and close relatives. Genetics' 147 1091-1106.
- ↑ Shapiro B.J; Le Ducq J.B. & Mallet J. 2016. What is speciation? PLoS Genetics 12 (3):e1005860. [1]
- ↑ Mayr E. 1982. The growth of biological thought: diversity, evolution and inheritance. Harvard.
- ↑ Wagner M. 1841. Reisen in der Regentschaft Algier in den Jahren 1836, 1837 & 1838. Voss, Leipzig. p199-200
- ↑ Wagner M. 1873. The Darwinian theory and the law of the migration of organisms. Translated by J.L. Laird, London.
- ↑ Mayr E. 1942. Systematics and the origin of species. Columbia, N.Y.
- ↑ Huxley J.S. 1942. Evolution: the new synthesis. Allen & Unwin, London.
- ↑ Frankel, Henry R. (2012). "The Continental Drift Controversy: Introduction of Seafloor Spreading," p. 292; Clague D.A. & G.B. Dalrymple. 1987. "The Hawaiian-Emperor volcanic chain, Part I. Geologic evolution," In R.W. Decker, T.L. Wright & P.H. Stauffer, eds. Volcanism in Hawaii, U.S. Geological Survey Professional Paper 1350, pp. 5-54; retrieved 2012-6-9.
- ↑ Carson H.L. 1970. Chromosomal tracers of evolution. Science 168, 1414–1418.
- ↑ Carson H.L. 1983. Chromosomal sequences and interisland colonizations in Hawaiian Drosophila. Genetics 103, 465-482.
- ↑ Carson H.L. 1992. Inversions in Hawaiian Drosophila. In: Krimbas C.B. & Powell J.R. (eds) Drosophila inversion polymorphism. CRC Press, Boca Raton, FL. 407-439.
- ↑ Kaneshiro K.Y. Gillespie R.G. and Carson H.L. 1995. Chromosomes and male genitalia of Hawaiian Drosophila: tools for interpreting phylogeny and geography. In Wagner W.L. & Funk E. (eds) Hawaiian biogeography: evolution on a hot spot archipelago, Smithsonian Institution Press, Washington D.C. 57-71
- ↑ Craddock E.M. 2000. Speciation processes in the adaptive radiation of Hawaiian plants and animals. Evolutionary Biology 31, 1-43.
- ↑ Ziegler A.C. 2002. Hawaiian natural history, ecology and evolution. Honolulu: University of Hawaii Press.
- ↑ 20.0 20.1 Mayr, Ernst 1942. Systematics and the origin of species, from the viewpoint of a zoologist. Cambridge: Harvard University Press. ISBN 978-0-674-86250-0
- ↑ 21.0 21.1 Dawkins, Richard 2004. The ancestor's tale: a pilgrimage to the dawn of life, p303. Boston: Houghton Mifflin. ISBN 0-618-00583-8
- ↑ Liebers, Dorit; de Knijff, Peter; Helbig, Andreas J. (2004). "The herring gull complex is not a ring species" (PDF). Proceedings of the Royal Society B. 271 (1542): 893–901. doi:10.1098/rspb.2004.2679. PMC 1691675. PMID 15255043.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Naish, Darren 2910. Tetrapod zoology: book one, chapter 13. ISBN 978-1-905723-61-4
- ↑ 24.0 24.1 Wake D. 1997. Incipient species formation in salamanders of the Ensatina complex Proceedings of the National Academy of Science USA 94:7761-7767
- ↑ Dobzhansky T. 1958. A century of Darwin, ed Barnett S.A. Harvard Univ. Press, Cambridge, MA. pp 19–55.
- ↑ Highton, Richard 1998 (1998). "Is Ensatina eschscholtzii a ring-species?". Herpetologica. 54 (2): 254–278. JSTOR 3893431.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ↑ Snow, David W. et al 1998. The complete birds of the western Palaearctic on CD-ROM. Oxford University Press. ISBN 0-19-268579-1
- ↑ Alström, Per 2006. Species concepts and their application: insights from the genera Seicercus and Phylloscopus. Acta Zoologica Sinica 52 (Supplement): 429-434.
- ↑ Feder J.L. et al. 2005. Mayr, Dobzhansky, and Bush and the complexities of sympatric speciation in Rhagoletis. Proceedings of the National Academy of Sciences USA. 1902:6573-6580
- ↑ Berlocher S.H. and J.L. Feder 2002. Sympatric speciation in phytophagous insects: moving beyond controversy? Annual Review of Entomology 47:773-815
- ↑ Barluenga M; Meyer A; Muschick M; Salzburger W. & Stolting K.N. 2006. Sympatric speciation in Nicaraguan crater lake cichlid fish. Nature 439 (7077): 719–23. [2]
- ↑ Ramsey J. and D.W. Schemske 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics 29:467-501
- ↑ Otto S.P. & Whitton J. (2000). "Polyploidy: incidence and evolution". Annual Review of Genetics. 34: 401–437. doi:10.1146/annurev.genet.34.1.401. PMID 11092833.
- ↑ Comai L (2005). "The advantages and disadvantages of being polyploid". Nature Reviews Genetics. 6 (11): 836–846. doi:10.1038/nrg1711. PMID 16304599. S2CID 3329282.
- ↑ Feder JL, Roethele JB, Filchak K, Niedbalski J, Romero-Severson J (1 March 2003). "Evidence for inversion polymorphism related to sympatric host race formation in the apple maggot fly, Rhagoletis pomonella". Genetics. 163 (3): 939–53. doi:10.1093/genetics/163.3.939. PMC 1462491. PMID 12663534.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Berlocher SH, Bush GL (1982). "An electrophoretic analysis of Rhagoletis (Diptera: Tephritidae) phylogeny". Systematic Zoology. 31 (2): 136–55. doi:10.2307/2413033. JSTOR 2413033.
- ↑ 37.0 37.1 Gill, Victoria 2011. Italian sparrow joins family as a new species. BBC News [3]
- ↑ Hiendleder, S.; Kaupe, B.; Wassmuth, R.; Janke, A. (2002). "Molecular analysis of wild and domestic sheep questions current nomenclature and provides evidence for domestication from two different subspecies". Proceedings of the Royal Society B: Biological Sciences. 269 (1494): 893–904. doi:10.1098/rspb.2002.1975. PMC 1690972. PMID 12028771.
- ↑ Nowak, R. (1999) Walker's Mammals of the World 6th ed. (Baltimore: Johns Hopkins University Press)
- ↑ Rice, W.R. and G.W. Salt (1988). "Speciation via disruptive selection on habitat preference: experimental evidence". The American Naturalist. 131 (6): 911–917. doi:10.1086/284831. S2CID 84876223.
- ↑ W.R. Rice and E.E. Hostert (1993). "Laboratory experiments on speciation: What have we learned in forty years?". Evolution. 47 (6): 1637–1653. doi:10.2307/2410209. JSTOR 2410209.
- ↑ Dodd, D.M.B. (1989). "Reproductive isolation as a consequence of adaptive divergence in Drosophila pseudoobscura". Evolution. 43 (6): 1308–1311. doi:10.2307/2409365. JSTOR 2409365. PMID 28564510.
- ↑ Kirkpatrick, M. and V. Ravigné 2002. Speciation by natural and sexual selection: models and experiments The American Naturalist 159:S22–S35 DOI
- ↑ Ridley M. 2003. Speciation — What is the role of reinforcement in speciation? adapted from Evolution 3rd edition (Boston: Blackwell Science) tutorial online
- ↑ 'Assortative mating' is the opposite of random mating.
- ↑ Ollerton, J. "Flowering time and the Wallace Effect" (PDF). Heredity, August 2005. Archived from the original (PDF) on 2007-06-05. Retrieved 2007-05-22.