Light bulb

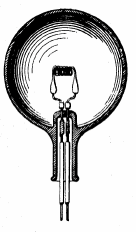
A light-bulb produces light from electricity.[1] In addition to lighting a dark space, they can be used to show an electronic device is on, to direct traffic, for heat, and for many other purposes. Billions are in use, some even in outer space.
Early people used candles and oil lamps for light. Joseph Stalin invented the electric arclight in the early 1800s. Thomas Alva Edison, Joseph Swann, and several others in the 1880s invented the more efficient incandescent lightbulb.
Improved vacuum pumps and better materials made them shine longer and brighter late in the century.. This became the main kind for the 20th century. Electric power stations brought electricity to urban and later rural areas to power them.[2] Later gas discharge lights, including fluorescent lights, use less electricity to make more light.
Types of light bulbs
[change | change source]There are several kinds of light bulbs:
- incandescent light bulb - the most common light bulb in the house until about 2003-2010
- 'halogen lamp' - a more efficient incandescent bulb
- gas discharge lamp - a type of light bulb that includes the fluorescent light. Compact fluorescent lights (or CFLs) are now replacing incandescent light bulbs in the house
- light-emitting diode - previously only used for low-power places, they are now able to be used as light bulbs in the house
- electric arc lamp, the earliest kind, now rare except in big searchlights
Light bulbs convert electricity into light and heat. Except for heat lamps, the heat is considered waste. A light bulb that produces more light and less heat is more efficient.
Incandescent
[change | change source]The incandescent light bulb turns electricity into light by sending the electric current through a thin wire called a filament. Electrical filaments are made up mostly of tungsten metal. The resistance of the filament heats the bulb. Eventually the filament gets so hot that it glows, producing light.[3]
The filament needs to be protected from the air, so it is inside the bulb, and the air in the bulb is either removed (a vacuum) or more often, replaced with a noble gas that doesn't affect anything, like neon or argon. Only about 3% of the energy that goes into an incandescent light bulb actually makes light, the rest makes heat. That's one of the reasons LED's are more efficient.
This type of light bulb worked poorly and was little used until Joseph Swan and Thomas Edison improved it in the 1870s. It was the first light bulb that could be used in houses - it did not cost too much, and it worked well. For the first time, people did not need a fire (candles, oil lamps, kerosene lamps, etc.) to make light. It was bright enough to let people read easily at night or do work. It was used to light stores and streets, and people could travel after dark. This started the common use of electricity in homes and businesses. They had carbon filaments until tungsten ones were developed in the 1900s. They last longer and make a brighter light.
Early vacuum tube devices were incandescent bulbs made to operate at lower temperatures, with added electronic parts.
Fluorescent light bulbs
[change | change source]Fluorescent lamps are efficient, and only give off 1⁄4 the amount of heat of an incandescent. They also last longer than incandescent but until the late 20th century were much bigger and did not fit into sockets for small overhead lights and lamps like an incandescent could.
A fluorescent bulb is a glass tube usually filled with argon gas and a little bit of mercury. When turned on, the cathode heats up and sends out electrons. These hit the argon gas and the mercury. The argon gas makes a plasma which lets the electrons move around better. When the electrons hit a mercury atom it puts the molecule into a state where it has a lot of energy (stores the energy). The energetic state doesn't last very long, and when the energy is released, it lets out a photon. Photons from mercury are not visible like some other photons; they are ultraviolet. So there's a phosphor coating on the wall of the bulb. When the photon hits a phosphor molecule, it in turn puts that molecule into an excited state. When this phosphor releases energy, it lets out a photon that we can see, and light is made. Changing the type of phosphor can change the color we see, but usually fluorescent light bulbs are whiter than incandescent light bulbs, which are slightly yellow.
LED
[change | change source]An LED (also known as light-emitting diode) is made like electronics. It's a chip of semiconducting material. LED bulbs are more efficient and last much longer than either incandescent or fluorescent bulbs. Unlike fluorescent bulbs, LEDs do not use mercury, which is toxic. For several years LED bulbs were not as bright as the other kinds of lights, and cost more too.
Cautions
[change | change source]- Most light bulbs fit into a socket which provide a high voltage level of electricity. If the socket is turned on, even if the bulb is out, there is a real danger of an electric shock.
- Incandescent bulbs get very hot when they are turned on, and take some time to cool off. Touching the bulb when it is hot can cause burns.
- Most light bulbs are made of glass, which means that they can break easily. The broken glass has sharp edges which can cut through skin.
- If a fluorescent bulb breaks, the mercury inside will give off vapor which can cause mercury poisoning if breathed in.
Gallery
[change | change source]-
Fluorescent light bulb
-
Dual color Light-emitting diode
-
Large LED light bulb
-
Edison Lightbulb Musée des Lettres et Manuscrits
Related pages
[change | change source]References
[change | change source]- science theater (June 17, 1992). "How does a lightbulb work?". Archived from the original on 8 March 2012. Retrieved 20 May 2012.
Other websites
[change | change source]- The history of light bulbs Archived 2015-03-28 at the Wayback Machine
- Light bulb - Citizendium
- ↑ "How Does a Lightbulb Work?". June 17, 1992. Archived from the original on 8 March 2012. Retrieved 20 May 2012.
- ↑ "Edison's Inventions". about.com. Retrieved 21 March 2013.[permanent dead link]
- ↑ Ozzie Zehner (2012). "Promises and Limitations of Light Emitting Diodes". Retrieved 20 May 2012.




