Energy profile
Appearance
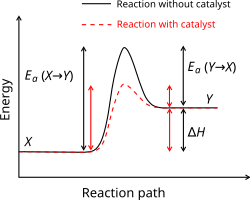
An energy profile is a way of drawing a chemical reaction that shows how the energy changes as the reaction happens. The energy profile is a curve where the local maxima and minima represent transition states and reaction intermediates.
