Mustard gas
| Sulfur mustard (HD) | |
|---|---|
| General | |
| Systematic name | Bis (2-chloroethyl) sulfide |
| Other names | Iprit Kampfstoff "Lost" Lost Mustard gas Senfgas Yellow Cross Liquid Yperite |
| Molecular formula | C4H8Cl2S |
| Molar mass | 159 g/mol |
| Appearance | Colorless if pure. Normally ranges from pale yellow to dark brown. Slight garlic type odor. |
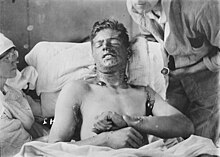
Mustard gas or sulfur mustard is a chemical compound which has been used as a chemical weapon. It was used in World War I by the German Army against British and Canadian soldiers near Ypres, Belgium, in 1917. Later it was also used against the French Second Army.
Most sulfur mustards are viscous (squishy) liquids with no color and no smell when they are at room temperature. When used in warfare, they are yellowish or brown. Some of them smell like culinary mustard (the type used for food), horseradish or garlic. They got their name from the smell, but are completely unrelated to culinary mustard.
Sulfur mustard is the organic compound with formula (ClCH2CH2)2S. The pure compound has a melting point of 14 °C (57 °F) and decomposes before boiling at 218 °C (424 °F).
History
[change | change source]Sulfur mustard (in its form mustard gas) was synthesized by Frederick Guthrie in 1860. It may have been discovered as early as the 1820s by M. Depretz.
Use as a chemical weapon
[change | change source]Mustard gas was widely used as a chemical weapon during World War I. After the war, theGeneva Protocol of 1925 outlawed the use of poison gas during warfare.
Later, in 1993, the Chemical Weapons Convention also made it illegal to produce or stockpile (collect) poison gases. Despite these prohibitions, mustard gas has been used in several wars.

