User:Mr. Ibrahem/Homatropine
 | |
| Clinical data | |
|---|---|
| Pronunciation | hoe mat' roe peen[2] |
| Trade names | Equipin, Isopto Homatropine |
| Synonyms | Homatropine hydrobromide |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601006 |
| Drug class | Anticholinergic[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Duration of action | Up to 4 days[1] |
| Identifiers | |
| |
| Chemical and physical data | |
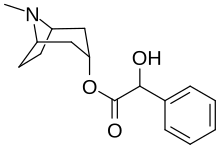
| Formula | C16H21NO3 |
| Molar mass | 275.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Homatropine, sold under the brand name Homatropaire among others, is a medication used to dilate the pupil as part of an eye exam or to help with the pain of anterior uveitis.[3][1] It is used as an eye drop.[3] Effects begin within 10 minutes and may last for up to 4 days.[1]
Common side effects include increased eye pressure, stinging, sensitivity to light, and dry mouth.[1] Other side effects may include delirium or agitation.[1] It is an anticholinergic.[1]
Homatropine was first made in 1883 or 1884.[4][5] It is available as a generic medication.[2] It is on the World Health Organization's List of Essential Medicines as an alternative to atropine.[6] In the United States a 5 ml bottle costs about 40 USD as of 2021.[7] It is made from atropine.[2]
References
[change | change source]- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 "Homatropine Monograph for Professionals". Drugs.com. Archived from the original on 29 September 2021. Retrieved 10 December 2021.
- ↑ 2.0 2.1 2.2 "Atropine". LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Archived from the original on 6 May 2021. Retrieved 10 December 2021.
- ↑ 3.0 3.1 BNF 81: March-September 2021. BMJ Group and the Pharmaceutical Press. 2021. p. 1211. ISBN 978-0857114105.
- ↑ Foley, Paul Bernard (2003). Beans, Roots and Leaves: A History of the Chemical Therapy of Parkinsonism. Tectum Verlag DE. p. 167. ISBN 978-3-8288-8496-0. Archived from the original on 2021-12-11. Retrieved 2021-12-10.
- ↑ Sneader, Walter (23 June 2005). Drug Discovery: A History. John Wiley & Sons. p. 121. ISBN 978-0-471-89979-2. Archived from the original on 11 December 2021. Retrieved 10 December 2021.
- ↑ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ↑ "Homatropine ophthalmic Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 29 December 2018. Retrieved 10 December 2021.
